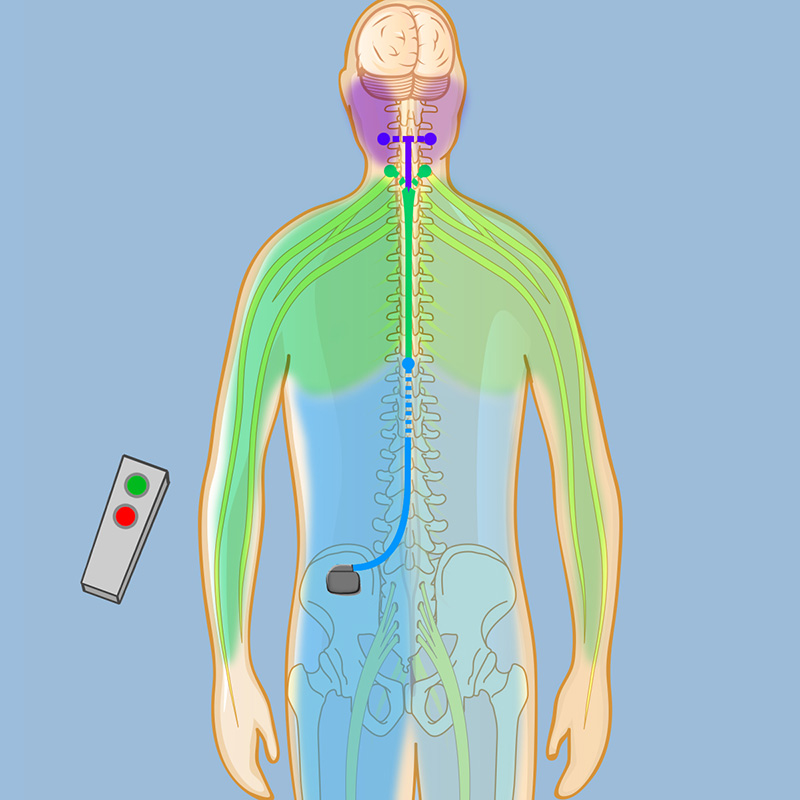
Neurosurgeon Michael D. Kaplitt, MD/PhD, and Pain Management specialist Neel Mehta, MD, recently implanted innovative new high-frequency spinal cord stimulators (SCSs) in two patients — the first in the tri-state area to receive them — for the relief of pain. This new device, which received FDA approval in May, represents a new generation of SCS technology and has the potential to deliver far better patient outcomes for those with chronic pain.
Existing SCS technology relieves pain by inducing a state of paresthesia — a steading tingling sensation that replaces the experience of pain. These low-frequency devices, although effective in many people, can be distracting in some patients — sudden movements can create inadvertent overstimulation — and they also don’t achieve the desired pain relief in everyone. The new high-frequency device can not only relieve pain, but it can do so without paresthesia or episodes of overstimulation.
The new high-frequency device, manufactured by Nevro, has been available for several years in Europe and has undergone extensive trials in the United States. Now FDA-approved, the device is a powerful treatment option for patients at the Weill Cornell Back and Neck Pain Program. (See more about the new device on the Pain Medicine web site.)
More about Spinal Cord Stimulation
More about the Weill Cornell Back and Neck Pain Program
More about Dr. Kaplitt
Request an Appointment | Refer a Patient
Illustration by Thom Graves, CMI