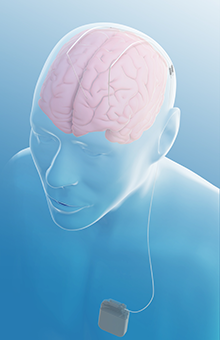
After more than a decade of testing, an advanced procedure for epilepsy is now available to patients whose seizures have not been successfully managed with medication. Deep brain stimulation (DBS), which has been effective for Parkinson’s disease and other movement disorders, was approved in 2018 by the Food and Drug Administration for medication-resistant epilepsy after a seven-year-long clinical trial. It is now commercially available in the United States in select locations, including the Weill Cornell Medicine Brain and Spine Center.
Dr. Michael Kaplitt, whose work throughout the clinical trial helped win FDA approval, was the first surgeon in New York to perform deep brain stimulation for epilepsy. The multi-center clinical trial, called Stimulation of the Anterior Nucleus of the Thalamus for Epilepsy (SANTE), tested the new device in more than 100 people over seven years; the median reduction in seizures was 75 percent. Study results were published in the journal Epilepsia (“Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy”) in 2010 reporting successful results from the first two years; a 2015 paper published in Neurology at five years concluded that “long-term follow-up of ANT deep brain stimulation showed sustained efficacy and safety in a treatment-resistant population,” and in 2018 the FDA approved the device for use in the United States. In early 2019 the device became commercially available, and the first insurance companies began providing coverage for the procedure.
“It’s extremely gratifying to see this procedure become available to patients,” says Dr. Kaplitt. “I was part of the study group for so many years, and I had become convinced that this was an effective way to help patients control seizures.” Dr. Kaplitt, an innovator in functional neurosurgery, was the first in the world to test gene therapy for Parkinson’s disease and in 2017 became the first neurosurgeon in New York to use focused ultrasound to treat essential tremor. (More about Dr. Kaplitt.)
“I was honored to participate in the FDA meeting that led to the approval of the device for epilepsy,” he says, “and I am pleased that this FDA approval now allows us to provide a minimally invasive intervention to greatly lessen seizures and vastly improve quality of life in patients with epilepsy.”
The technology, made by Medtronic, consists of the same DBS device currently used for movement disorders plus an external patient programmer that patients can use to control the stimulation. The device is implanted in the chest below the collar bone and delivers electrical stimulation over tiny wires to the anterior nucleus of the thalamus (ANT), interrupting the propagation of seizures. (The electrodes can be directed to very specific points in the brain, which is why this process works on multiple conditions; different parts of the brain can be stimulated to relieve the symptoms of Parkinson’s disease, essential tremor, and now epilepsy.)
Deep brain stimulation now joins other minimally invasive neurosurgical options for patients with epilepsy that does not respond to medication. Weill Cornell Medicine is one of the few places in the United States to offer a wide range of options for patients with epilepsy, including traditional neurosurgery, laser surgery, and neurostimulation therapy–which includes vagal nerve stimulation (VNS), responsive neurostimulation (RNS), and now deep brain stimulation (DBS).
For more information about DBS for epilepsy, please contact Dr. Kaplitt’s office (212-746-4966) or use the online Request an Appointment form.